Healthy coral reefs are one of the most valuable ecosystems on Earth. They provide billions of dollars in economic and environmental services, such as food, coastal protection, and tourism. However, coral ecosystems around the world face serious threats from a number of sources, including climate change, unsustainable fishing, land-based pollution, coastal development, disease, and invasive species.
Scientists have also discovered that some of the chemicals found in sunscreen and other personal health products threaten the health of coral reefs. How these, and other compounds, affect reef ecosystems remains an active area of research. In August 2022, the National Academy of Sciences released a study which reviews the state of the science on the use of sunscreen ingredients and their environmental impacts. Overall the study found that specific (chemical) UV filters found in chemical sunscreen can harm aquatic life, including corals, and therefore, a more comprehensive ecological risk assessment of all UV filters in chemical sunscreen is needed. Mineral sunscreen, which does not use (chemical) UV filters, is considered a better option because there are less effects to aquatic organisms. Wearing UV protective clothing, like sun shirts and pants, is also a great option.
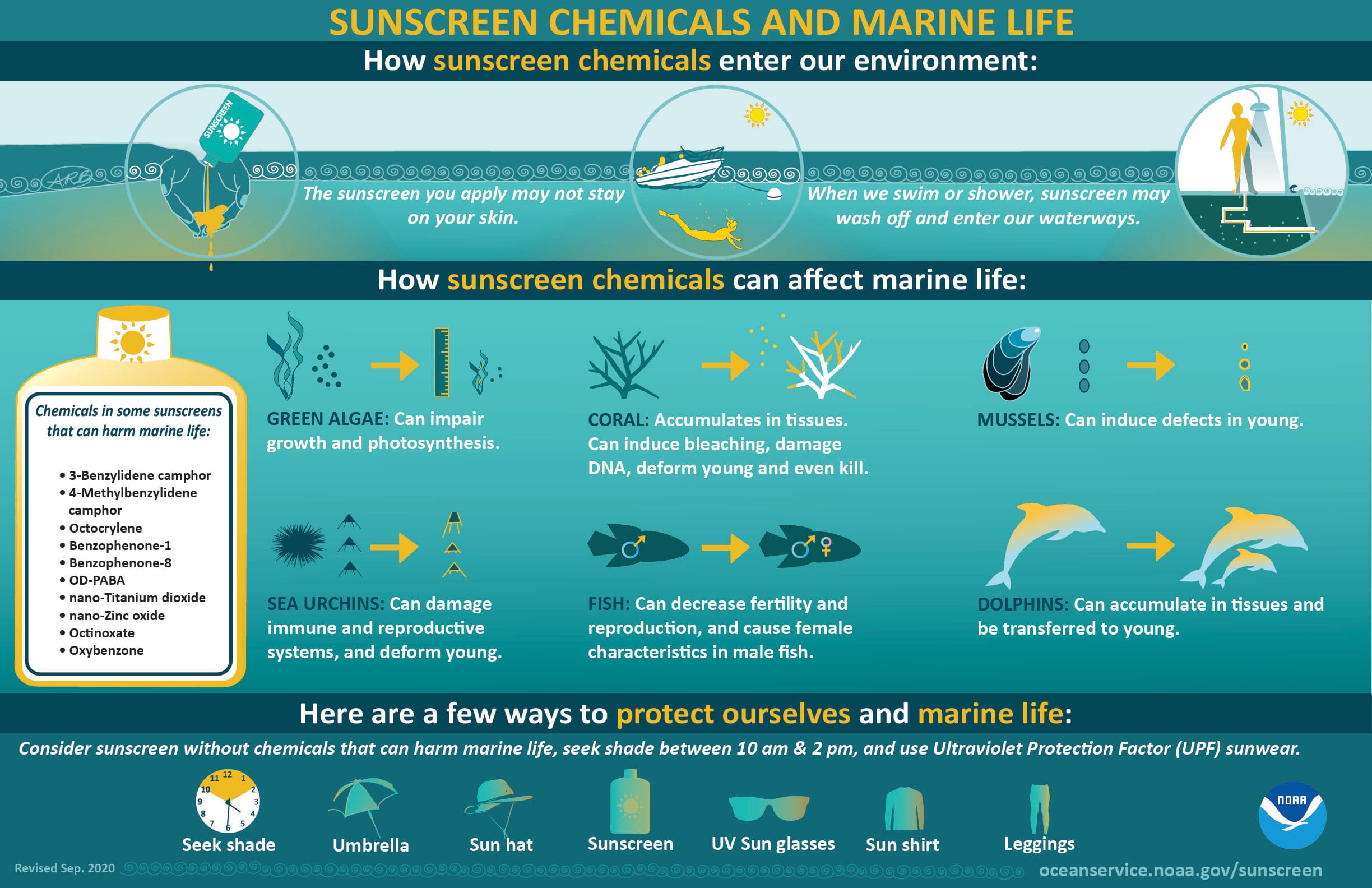
Infographic Transcript: Sunscreen Chemicals and Marine Life
- How sunscreen chemicals enter our environment: The sunscreen you apply may not stay on your skin. When we swim or shower, sunscreen may wash off and enter our waterways.
- How sunscreen chemicals can affect marine life:
- Green Algae: Can impair growth and photosynthesis.
- Coral: Accumulates in tissues. Can induce bleaching, damage DNA, deform young, and even kill.
- Mussels: Can induce defects in young.
- Sea Urchins: Can damage immune and reproductive systems, and deform young.
- Fish: Can decrease fertility and reproduction, and cause female characteristics in male fish.
- Dolphins: Can accumulate in tissue and be transferred to young.
- Chemicals in some sunscreens that can harm marine life include: Oxybenzone, Benzophenone-1, Benzophenone-8, OD-PABA, 4-Methylbenzylidene camphor, 3-Benzylidene camphor, nano-Titanium dioxide, nano-Zinc oxide, Octinoxate, Octocrylene
- Here are a few ways to protect ourselves and marine life: Consider sunscreen without chemicals that can harm marine life, seek shade between 10 am & 2 pm, and use Ultraviolet Protection Factor (UPF) sunwear.
 An official website of the United States government.
An official website of the United States government.

Social